What is tarnish and what causes it?
The chemical name given to the tarnishing process is oxidation. Technically, oxidation means the loss of an electron. In our case this process results in the formation of a dark brown to black discolouration on the surface of silver. It is largely the result of hydrogen sulphide reacting with the metal forming a sulphide. Other sulphur compounds will also attach themselves to the silver with the same result.
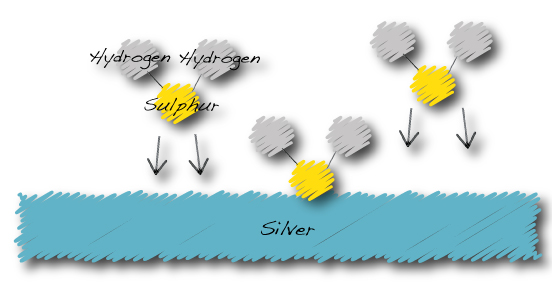
Hydrogen Sulphide in the atmosphere is attracted to the surface of silver…
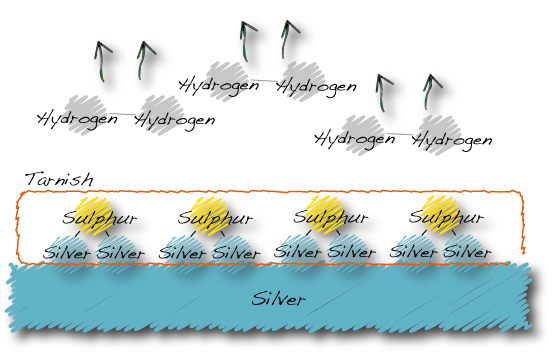
The silver tarnishing process:
…Hydrogen sulphide becomes silver sulphide and hydrogen gas.
The silver sulphide is the grey-brown-black coating we call tarnish.
In addition to silver, this tarnishing process will occur on low carat gold and other metals too. The surface of the metal will loose its lustre and together with oil and dirt, tarnish will make the metal look in need of a good polish.
Re-polishing is the usual remedy which is time consuming, risky to stones and their settings, dirty, unpleasant, but it can do unnecessary damage to the surface of the metal and stones. Each time the piece is re-polished, a small amount of metal is removed. This is terminally damaging for claw or bevel settings as the metal will be weakened. Each time it is done it brings the day a stone drops out a little closer. Jewellery set with delicate stones such as opal, pearl require great care to be exercised so that the polished surface of the stone is not marred. This can turn out to be very expensive. This was the key reason we designed the JCR cleaning system.
How to minimise (slow down) jewellery tarnishing
The tarnishing process is unfortunately inevitable with silver and low carat gold items, however there are steps that can be taken to slow down the process. This is particularly useful for jewellery retailers who may have large collections of silver jewellery on display.
Environmental Causes
Sulphur and oxygen is the primary cause of gold and silver jewellery tarnish. Unfortunately some areas can have naturally elevated levels of sulphur such as areas with high levels of traffic (exhaust gas), areas of heavy industry (air pollution), and areas with volcanic vents – such as hot springs and geysers. In such areas the options are extremely limited, but the following tips may help prolong the intervals between cleans.
- High levels of humidity and heat will further accelerate the tarnishing process. If your shop is not already air-conditioned, try installing a dehumidifier.
- If the display jewellery is inside an illuminated display cabinet make sure that the lighting uses LEDs. These produce far less heat than halogen and other incandescent forms of lighting.
- Try to keep jewellery handling to a minimum.
Jewellery display cabinet
The jewellery display cabinet itself can be a significant and unexpected cause of accelerated tarnishing. This can be caused by synthetic woods such as MDF and particle board, paints, adhesives, varnishes, glues, foams and fabrics releasing gas as they age (out-gassing). This is a particular problem with brand new cabinets – the level of out-gassing tends to be at it’s highest directly after manufacture. If you have a brand new cabinet it is well worth leaving the glass doors open in a warm dry environment for several days to release the gas before it is stocked with jewellery. In custom fit-outs the varnishes and paints can take several weeks to fully cure, and some (particularly two pack epoxy finishes) may emit chemicals such as formaldehyde.
Display cabinets are generally enclosed and illuminated with bright lighting. The lighting causes the internal temperature of the cabinet to rise, this will elevate the materials natural tendency to outgass. Further to this, the tarnishing process is also accelerated by heat. Examine the type of lighting used – this should ideally be cool running LEDs. Always avoid using tungsten or halogen lighting – these tend to emit more heat than light!
Retail Packaging, pouches, paper and plastic bags
The choice of materials used to make retail packaging can sometimes be the source of tarnishing problems caused by out-gassing with some plastics, glues, foams and fabrics. If custom packaging is used, when selecting, changing manufacturers or brands it is always worth taking the time to run experiments and take note of how long it takes items to tarnish before committing to bulk orders. This will vary, so always test your packaging!
Resealable plastic bags are often considered to be an excellent way of retarding tarnishing – however not all bags are suitable. The worst case is when the plastic is made of or contains PVC. This tends to emit chlorine which will rapidly cause items to tarnish. The best type of plastic bag for storing jewellery is made from low density polyethylene (LDPE). Bubble wrap should also be avoided. It is also worth checking that the bag seals properly. This can be performed by blowing air inside the bag, sealing it and compressing it to check it does not leak – don’t store jewellery in the bag you have just blown in to!
Tissue paper and paper bags can be surprisingly problematic. Some paper emits sulphur, and can also be acidic. It is best to avoid wrapping items in paper, however paper or tissue can be used to help protect items that are already sealed and protected inside LDPE bags.
Labels and tags need to be treated with caution as they may contain adhesives, acids or PVC that can emit chlorine, formaldehyde or sulphur. It is worth conducting experiments by sealing two similarly polished items in separate sealed LDPE bags with one containing the price tag or label and comparing the rate of tarnish. A sealed jewellery cabinet containing dozens of problematic labels or tags has the potential to be a major source of tarnishing problems.
Handling
Regularly handling jewellery will also speed up tarnishing. This can be a particular problem with individuals on certain medications. Unfortunately handling jewellery tends to be unavoidable in a retail environment, but whenever possible use cotton gloves to handle jewellery directly after it has been cleaned, and when organising or arranging a retail display.
Storing jewellery to minimise tarnishing
Sometimes it is necessary to store an item of jewellery for a prolonged period. In order to do this and preserve the lustre for as long as possible the following technique can be helpful:
- 1) Remove any price tags or labels that may be attached to the item.
- 2) Clean the item of jewellery with a fresh mix of Ion-Sol and a JCR cleaner,
- 3) Rinse with distilled water, then pat dry with a clean lint free cotton cloth.
- 4) Only handle the jewellery with fresh cotton gloves, and place the freshly cleaned and thoroughly dry item inside a sealed polyethylene (LDPE) plastic bag.
- 5) Never blow inside the bag to open it!
- 6) Try to squash out as much of the air from inside the bag as possible before it is sealed.
Wearing jewellery
When wearing jewellery it will inevitably loose it’s lustre. To preserve it for as long as possible avoid wearing the items in pools or spars. The chlorine and bromine will rapidly cause tarnish – and the resulting type of tarnish can be quite hard to remove, often requiring abrasive methods. Also avoid wearing jewellery when cleaning – especially when using reactive chemicals such as bleach.
